PESG Research Update: PolyPid SHIELD’s II Delivers Transformative Phase 3 Topline Results - Paradigm Shift in Surgical Infection Prevention
PESG issues a new briefing following PolyPid’s clinical success; Revolutionary 58% Reduction in Surgical Site Infections Positions PolyPid’s D-PLEX100 as Potential New Standard of Care in the Future
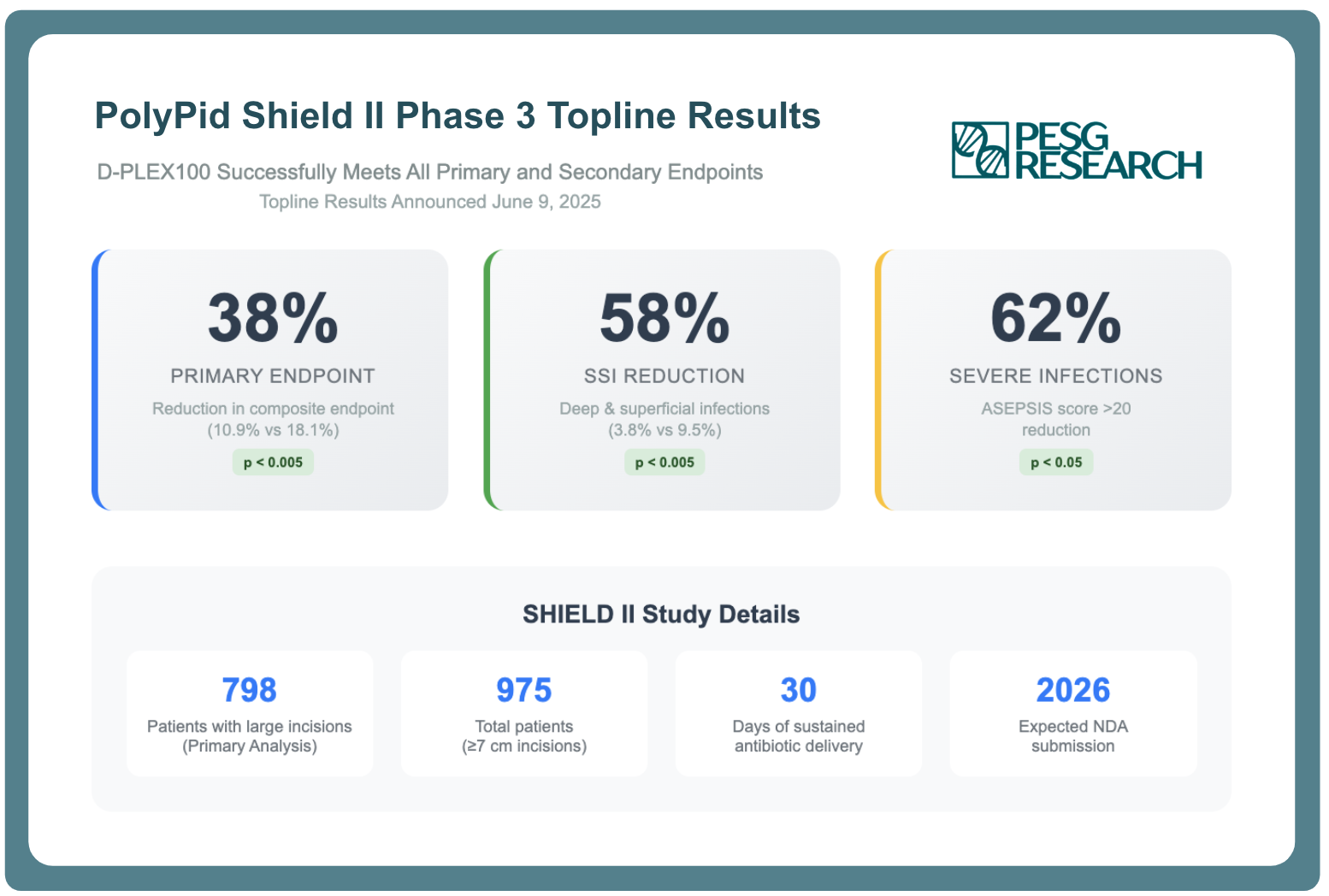
On June 9th, PolyPid Ltd.* announced landmark topline results from its pivotal SHIELD II Phase 3 trial, demonstrating that D-PLEX100 achieved statistically significant reductions across all primary and secondary endpoints in preventing surgical site infections (SSIs).
The study's compelling efficacy profile—including a 58% reduction in SSIs, 38% reduction in the composite primary endpoint, and 62% reduction in severe wound infections—validates the company's innovative PLEX technology platform and positions D-PLEX100 as a potential paradigm shift in surgical infection prevention.
These results represent what seems like clinical an unpralleled clinical breakthrough in addressing one of healthcare's most persistent challenges, with implications extending far beyond the annual US healthcare costs of SSI which have been estimated at $10 billion. The successful demonstration of localized, sustained antibiotic delivery directly at surgical sites opens new possibilities for infection prevention strategies while potentially reducing the systemic antibiotic burden that contributes to antimicrobial resistance.
New York, June 11, 2025 (GLOBE NEWSWIRE) -- PESG Research is releasing a briefing today examining the groundbreaking Phase 3 topline results recently published by PolyPid Ltd. This briefing contains partner content, please refer to the disclaimers and disclosures included at the end of it. The full briefing is available below.
SHIELD II: Definitive Evidence of Clinical Efficacy
The SHIELD II trial delivered unequivocal evidence of D-PLEX100's clinical utility across multiple measures of surgical outcomes. In the 798-patient cohort with large abdominal incisions, D-PLEX100 plus standard of care achieved:
- Primary Endpoint Success: The composite primary endpoint of SSIs, all-cause mortality, and surgical reinterventions was met with a 38% reduction in events (10.9% vs 18.1% in the control arm, p<0.005). This represents a clinically meaningful improvement that addresses the most significant complications following colorectal surgery.
- Dramatic SSI Reduction: The 58% reduction in deep and superficial SSIs (3.8% vs 9.5% in the control arm, p<0.005) moves the infection rate with D-PLEX100 treatment below what surgeons typically observe even in lower-risk procedures. This level of reduction is particularly noteworthy given that the study population comprised high-risk patients with large surgical incisions.
- Broad Efficacy Across Populations: The second key secondary endpoint demonstrated efficacy across the broader study population of 975 patients with incisions ≥7 cm, including laparoscopic surgery patients, suggesting D-PLEX100's benefits may extend beyond the initial target population.
- Severe Infection Prevention: The 62% reduction in patients with ASEPSIS scores >20 indicates D-PLEX100's particular effectiveness in preventing the most severe wound infections that typically require intensive interventions and extended hospitalizations.
Further Scientific Validation of PLEX Technology Platform
The SHIELD II topline results provide important validation of PolyPid's proprietary PLEX (Polymer-Lipid Encapsulation matriX) technology. The platform's ability to deliver sustained, localized antibiotic concentrations for 30 days directly at surgical sites has now been demonstrated to translate into clinically meaningful outcomes.
The technology's sophisticated layered matrix structure enables precise control of doxycycline release kinetics, achieving high local antibiotic concentrations while using only a fraction of the total drug amount (D-PLEX100 uses 55-164 mg of doxycycline compared to 6,000 mg in systemic formulations). This approach addresses the fundamental limitation of current prophylactic strategies, where systemic antibiotics face significant barriers to adequate tissue penetration at surgical sites due to disrupted blood flow.
The broad-spectrum activity of doxycycline against both gram-positive and gram-negative bacteria, including antibiotic-resistant strains such as MRSA, positions D-PLEX100 to address the evolving landscape of surgical pathogens. The localized delivery mechanism may also help preserve the effectiveness of systemic antibiotics by reducing selective pressure for resistance development.
Market Impact and Healthcare Economics
The SHIELD II results have profound implications for healthcare economics and surgical practice patterns. SSIs currently affect up to 30% of colorectal surgeries, leading to extended hospital stays averaging 7-11 additional days and imposing direct costs of $11,000-26,000 per infection. The demonstrated 58% reduction in SSI rates could translate to substantial healthcare cost savings across the target population.
- The Surgical Landscape: PolyPid's market research indicates approximately 4.4 million soft-tissue surgeries annually could benefit from D-PLEX100, encompassing hernia repairs, appendectomies, and colorectal surgeries. The company targets a total addressable U.S. market of over 12 million annual surgeries. The technology's demonstrated efficacy across different surgical contexts suggests potential for label expansion beyond the initial colorectal indication.
- Post-COVID Infection Trends: The CDC has documented a 3% increase in SSI rates following the COVID-19 pandemic, potentially reflecting changes in patient acuity, surgical case mix, or healthcare delivery patterns. D-PLEX100's ability to reduce infection rates to below historical norms positions it as a valuable tool for addressing these evolving challenges.
- Partnership Interest: Management has reported increased interest from potential commercialization partners following these positive results, with PolyPid seeking collaborations with companies having established hospital relationships and SSI prevention expertise. The company's existing European partnership with Advanz Pharma provides a template for global commercialization strategies.
Regulatory Pathway and Timeline
The SHIELD II results position PolyPid for regulatory submissions in early 2026, with both FDA New Drug Application (NDA) and European Medicines Agency Marketing Authorization Application (MAA) filings planned. The FDA's previous assignment of Breakthrough Therapy, Fast Track, and Qualified Infectious Disease Product (QIDP) designations provides multiple pathways for expedited review and approval.
The QIDP designation offers particular advantages, including potential priority review, extended market exclusivity, and eligibility for additional FDA meetings during the review process. These incentives reflect regulatory recognition of the critical need for new approaches to combat antimicrobial resistance and healthcare-associated infections.
Broader Platform Implications
Beyond the immediate D-PLEX100 opportunity, the SHIELD II results validate PolyPid's PLEX technology platform for broader therapeutic applications. The company's OncoPLEX program, currently in preclinical development for localized chemotherapy delivery in solid tumors, represents a logical extension of the proven controlled-release technology.
The platform's versatility in delivering different active pharmaceutical ingredients with precise release kinetics opens possibilities for applications in various therapeutic areas where localized, sustained drug delivery offers advantages over systemic administration. The successful demonstration of clinical efficacy in SHIELD II provides a strong foundation for these future developments.
Outlook
The SHIELD II Phase 3 topline results represent a potential watershed moment in surgical infection prevention, providing definitive evidence that localized, sustained antibiotic delivery can dramatically reduce SSI rates while maintaining an acceptable safety profile. The 58% reduction in surgical site infections, combined with broad efficacy across multiple endpoints, positions D-PLEX100 as a potential new standard of care for high-risk surgical procedures.
These results provide further validation not only D-PLEX100's clinical utility but also the broader PLEX technology platform, opening new possibilities for addressing unmet medical needs across multiple therapeutic areas. As PolyPid advances toward regulatory submissions and commercialization partnerships, the SHIELD II data provides a compelling foundation for transforming surgical practice and improving patient outcomes on a global scale.
The convergence of demonstrated clinical efficacy, substantial market opportunity, and regulatory support positions D-PLEX100 as one of the most significant advances in surgical infection prevention in decades. Healthcare providers, payers, and patients all stand to potentially benefit from this innovative approach to a persistent clinical challenge.
Read PolyPid’s announcement with further details about the resutls: PolyPid Announces Positive Topline Results from Phase 3 SHIELD II Trial: D-PLEX₁₀₀ Demonstrated Significant Reduction in Surgical Site Infections and Successfully Met Primary and All Key Secondary Endpoints
>> Click here to Subscribe for more updates like this or go to https://www.pesgresearch.com/subscribe
* Important Disclaimers & Disclosures: Nothing in this report constitutes medical, financial or any form professional or licensed advice. This report is published by 'PESG Research', a digital promotional content brand which is part of the wall street wire network of brands, whose operators are compensated to provide digestible and favorable coverage of companies. This report contains and is a form of paid promotional content or advertising for PolyPid Ltd. This report has not been reviewed or approved by PolyPid prior to publication and it does not represent an official communication from PolyPid. PESG and Wall Street Wire’s operators, Arx Advisory Ltd, the operators of PESG Research, received or are expected to receive a monthly recurring subscription fee of five thousand United States dollars via wire transfer from PolyPid Ltd as part of an ongoing agreement starting May 1, 2025 in return for distribution and promotional coverage services (as part of their "Wall Street Wire" platform), and receive additional monthly compensation for advisory, monitoring and unrelated data services on top of that. Please review the full disclaimers and compensation disclosures here for further details: redditwire.com/terms. Readers are advised to refer to the official materials of the company aforementioned. The report should not be treated as objective.

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
